The efficacy of bacteriophages in destroying biofilms on urinary catheters in experimental in vitro and in vivo models
- Authors: Aslanov B.I.1,2, Konev S.D.3, Makarova M.A.1,4, Gadzhiev N.K.3, Gorgotsky I.A.3, Kulyash A.G.3, Rozhkovan K.V.3, Vasyutina M.L.5, Murashova L.A.5, Loshachenko A.S.3, Bryukhanova V.V.3, Rybalchenko O.V.3, Bondarenko A.S.6, Konstantinova A.M.3, Galkovsky B.E.3
-
Affiliations:
- North-Western State Medical University named after I.I. Mechnikov
- Smorodintsev Research Institute of Influenza
- Saint Petersburg State University
- Saint Petersburg Pasteur Institute
- Almazov National Medical Research Centre
- Scientific Technologies and Service LLC
- Issue: Vol 102, No 6 (2025)
- Pages: 702-718
- Section: ORIGINAL RESEARCHES
- URL: https://medbiosci.ru/0372-9311/article/view/381676
- DOI: https://doi.org/10.36233/0372-9311-731
- EDN: https://elibrary.ru/FICDRG
- ID: 381676
Cite item
Abstract
Introduction. The formation of biofilms by healthcare-associated infection (HAI) pathogens on invasive medical devices is an increasingly urgent problem in clinical practice. Microbial biofilms contribute to persistent infections, complicate treatment, increase healthcare costs, and reduce the quality of patient care. The resistance of biofilm-embedded bacteria to antibiotics is a key factor in chronic and recurrent infections. In this context, bacteriophages may serve as a promising therapeutic agent against bacterial infections, including those caused by biofilm-forming microorganisms.
Objective. To assess the efficacy of lytic bacteriophages in disrupting microbial biofilms on urinary catheters using in vitro and in vivo experimental models.
Materials and methods. The study employed microbiological, morphological, and electron microscopy techniques. In vitro, biofilms were cultured on urinary catheter surfaces and subsequently treated with bacteriophages. For the in vivo model, catheter-associated urinary tract infection (CAUTI) was induced in mice, which were then divided into experimental and control groups. Bacteriophages were administered transurethrally.
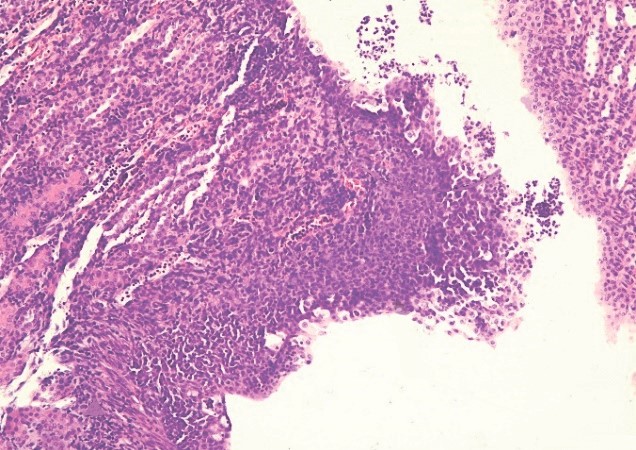
Results. In the in vitro model, bacteriophages effectively disrupted biofilms, inducing bacterial cell lysis and degradation of the exopolysaccharide matrix. In the in vivo experiments, mice treated with bacteriophages exhibited regression of CAUTI, as confirmed by morphological and bacteriological analyses. Electron microscopy revealed biofilm destruction on 5 out of 6 catheters. In contrast, the positive control group showed progressive infection, while no biofilm formation was observed in the negative controls.
Conclusions. The findings from both in vitro and in vivo experiments demonstrate that bacteriophages are capable of degrading biofilms and may represent an effective therapeutic strategy against biofilm-associated HAIs.
About the authors
Batyrbek I. Aslanov
North-Western State Medical University named after I.I. Mechnikov; Smorodintsev Research Institute of Influenza
Email: batyrbek.aslanov@szgmu.ru
ORCID iD: 0000-0002-6890-8096
Dr. Sci. (Med.), Professor, Director, Institute of Preventive Medicine, Head, Department of epidemiology, parasitology and disinfection, North-Western State Medical University named after I.I. Mechnikov; leading researcher, Biotechnology depatment, Smorodintsev Research Institute of Influenza
Russian Federation, St. Petersburg; St. PetersburgSergei D. Konev
Saint Petersburg State University
Author for correspondence.
Email: sd-konev@yandex.ru
ORCID iD: 0000-0003-1919-4725
Head, Epidemiology department, N.I. Pirogov Clinic of High Medical Technologies
Russian Federation, St. PetersburgMaria A. Makarova
North-Western State Medical University named after I.I. Mechnikov; Saint Petersburg Pasteur Institute
Email: makmaria@mail.ru
ORCID iD: 0000-0003-3600-2377
Dr. Sci. (Med.), Associate Professor, leading researcher, Head, Laboratory of intestinal infections, Saint Petersburg Pasteur Institute; Professor, Department of medical microbiology, North-Western State Medical University named after I.I. Mechnikov
Russian Federation, St. Petersburg; St. PetersburgNariman K. Gadzhiev
Saint Petersburg State University
Email: nariman.gadjiev@gmail.com
ORCID iD: 0000-0002-6255-0193
D. Sci. (Med.), urologist, deputy chief physician, Medical department (Urology), N.I. Pirogov Clinic of High Medical Technologies; Professor, Urology department, Medical Institute, Saint Petersburg State University
Russian Federation, St. PetersburgIvan A. Gorgotsky
Saint Petersburg State University
Email: igorgotsky@gmail.com
ORCID iD: 0000-0002-8514-5510
Cand. Sci. (Med.), urologist, deputy chief physician for outpatient clinical care, N.I. Pirogov Clinic of High Medical Technologies; Associate Professor, Urology department, Medical Institute, Saint Petersburg State University
Russian Federation, St. PetersburgAlexey G. Kulyash
Saint Petersburg State University
Email: kulyash_patolog@bk.ru
ORCID iD: 0000-0002-9916-6232
Head, Laboratory of molecular genetic research, N.I. Pirogov Clinic of High Medical Technologies
Russian Federation, St. PetersburgKonstantin V. Rozhkovan
Saint Petersburg State University
Email: tomcat-27@yandex.ru
ORCID iD: 0000-0002-8403-8342
Cand. Sci. (Biol.), biologist, Laboratory of molecular genetic research, N.I. Pirogov Clinic of High Medical Technologies
Russian Federation, St. PetersburgMarina L. Vasyutina
Almazov National Medical Research Centre
Email: raluwow@gmail.com
ORCID iD: 0000-0002-3295-8411
researcher, Laboratory of bioprosthetics and cardioprotection, Institute of Experimental Medicine
Russian Federation, St. PetersburgLada A. Murashova
Almazov National Medical Research Centre
Email: barbosachka85@gmail.com
ORCID iD: 0000-0001-7155-1078
junior researcher, Research on neurogenesis and neurodegenerative diseases
Russian Federation, St. PetersburgAnton S. Loshachenko
Saint Petersburg State University
Email: a.loshachenko@spbu.ru
ORCID iD: 0000-0002-1058-3452
Cand. Sci. (Phys., Math.), Director, Interdisciplinary Resource Center for Nanotechnology
Russian Federation, St. PetersburgVera V. Bryukhanova
Saint Petersburg State University
Email: verabryu@gmail.com
ORCID iD: 0000-0002-9862-1387
engineer, Interdisciplinary Resource Center for Nanotechnology
Russian Federation, St. PetersburgOksana V. Rybalchenko
Saint Petersburg State University
Email: o.rybalchenko@spbu.ru
ORCID iD: 0000-0001-9758-0053
Dr. Sci. (Med.), Professor, Department of physiology
Russian Federation, St. PetersburgAnton S. Bondarenko
Scientific Technologies and Service LLC
Email: bond.anton@gmail.com
ORCID iD: 0000-0001-7707-1710
Cand. Sci. (Phys., Math.), technical director
Russian Federation, ChernogolovkaAnastasia M. Konstantinova
Saint Petersburg State University
Email: anastasia.konstantynova@gmail.com
ORCID iD: 0000-0002-2595-2249
Dr. Sci. (Med.), Associate Professor, Head, Department of pathology, N.I. Pirogov Clinic of High Medical Technologies
Russian Federation, St. PetersburgBoris E. Galkovsky
Saint Petersburg State University
Email: mrc4se@gmail.com
ORCID iD: 0000-0002-5252-483X
Cand. Sci. (Med.), pathologist, Department of pathology, N.I. Pirogov Clinic of High Medical Technologies
Russian Federation, St. PetersburgReferences
- Chinemerem Nwobodo D., Ugwu M.C., Oliseloke Anie C., et al. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022;36(9):e24655. DOI: https://doi.org/10.1002/jcla.24655
- Assefa M., Amare A. Biofilm-associated multi-drug resistance in hospital-acquired infections: a review. Infect. Drug. Resist. 2022;15:5061–8. DOI: https://doi.org/10.2147/IDR.S379502
- Sharma S., Mohler J., Mahajan S.D., et al. Microbial biofilm: a review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms. 2023;11(6): 1614. DOI: https://doi.org/10.3390/microorganisms11061614
- Shree P., Singh C.K., Sodhi K.K., et al. Biofilms: understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 2023;16(5):100084. DOI: https://doi.org/10.1016/j.medmic.2023.100084
- Grooters K.E., Ku J.C., Richter D.M., et al. Strategies for combating antibiotic resistance in bacterial biofilms. Front. Cell Infect. Microbiol. 2024;14:1352273. DOI: https://doi.org/10.3389/fcimb.2024.1352273
- Конев С.Д., Асланов Б.И., Ширай О.В. и др. Эпидемиологическая характеристика инфекций, вызванных биопленочными формами микроорганизмов, у пациентов с инвазивными медицинскими устройствами. Инфекционные болезни. 2025;23(2):53–60. Konev S.D., Aslanov B.I., Shirai O.V., et al. Epidemiological characterization of infections caused by biofilm-forming microorganisms in patients with invasive medical devices. Infectious Diseases. 2025;23(2):53–60. DOI: https://doi.org/10.20953/1729-9225-2024-4-21-25 EDN: https://elibrary.ru/lafcms
- Jamal M., Ahmad W., Andleeb S., et al. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018;81(1):7–11. DOI: https://doi.org/10.1016/j.jcma.2017.07.012
- Stewart P.S., Bjarnsholt T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infect. 2020;26(8):1034–8. DOI: https://doi.org/10.1016/j.cmi.2020.02.027
- Muteeb G., Rehman M.T., Shahwan M., Aatif M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: a narrative review. Pharmaceuticals (Basel). 2023;16(11):1615. DOI: https://doi.org/10.3390/ph16111615
- Gliźniewicz M., Miłek D., Olszewska P., et al. Advances in bacteriophage-mediated strategies for combating polymicrobial biofilms. Front. Microbiol. 2024;14:1320345. DOI: https://doi.org/11.3389/fmicb.2023.1320345
- Liu S., Lu H., Zhang S., et al. Phages against pathogenic bacterial biofilms and biofilm-based infections: a review. Pharmaceutics. 2022;14(2):427. DOI: https://doi.org/10.3390/pharmaceutics14020427
- O'Toole G.A., Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 1998;28(3):449–61. DOI: https://doi.org/10.1046/j.1365-2958.1998.00797.x
- Конев С.Д. Адаптированный метод выявления биопленок на инвазивных устройствах, применяемых в урологической практике. Материалы конференции «Эйхвальдские чтения-2023». СПб.;2023. Konev S.D. An adapted method for detecting biofilms on invasive devices used in urological practice. In: Proceedings of the Conference «Eichwald Readings-2023». St. Petersburg;2023. EDN: https://elibrary.ru/cztntv
- Conover M.S., Flores-Mireles A.L., Hibbing M.E., et al. Establishment and characterization of UTI and CAUTI in a mouse model. J. Vis. Exp. 2015;23:(100):e52892. DOI: https://doi.org/10.3791/52892
- Асланов Б.И., Конев С.Д., Куляш А.Г. и др. Метод быстрой идентификации биопленок на инвазивных устройствах, применяемых в урологической практике. В кн.: Профилактическая медицина — 2022: сборник научных трудов Всероссийской научно-практической конференции с международным участием. СПб.;2022:22–6. Aslanov B.I., Konev S.D., Kulyash A.G., et al. Method fast identification of biofilms on invasive devices used in urological practice. In: Preventive Medicine — 2022: Collection of Scientific Papers of the All-Russian Scientific and Practical Conference with the Participation. St. Petersburg; 2022:22–6. EDN: https://elibrary.ru/zdwtqc
- Dagnaes-Hansen F., Kilian M., Fuursted K. Septicaemia associated with an Aerococcus viridans infection in immunodeficient mice. Lab. Anim. 2004;38(3):321–5. DOI: https://doi.org/10.1258/002367704323133718
- Gryaznova M., Smirnova Y., Burakova I., et al. Effect of probiotic bacteria on the gut microbiome of mice with lipopolysaccharide-induced inflammation. Microorganisms. 2024;12(7):1341. DOI: https://doi.org/10.3390/microorganisms12071341
- Zalewska-Piątek B. Phage therapy — challenges, opportunities and future prospects. Pharmaceuticals (Basel). 2023; 16(12):1638. DOI: https://doi.org/10.3390/ph16121638
- Li X., He Y., Wang Z., et al. A combination therapy of phages and antibiotics: two is better than one. Int. J. Biol. Sci. 2021;17(13):3573–82. DOI: https://doi.org/10.7150/ijbs.60551
- Gomes Dallepiane F., Alejandro Coimbra Nogueira M., Menezes Dos Anjos L., et al. Bacteriophages as potential therapeutic agents in the control of bacterial infections. EXCLI J. 2025;24:524–6. DOI: https://doi.org/10.17179/excli2025-8145
- Tian F., Li J., Nazir A., et al. Bacteriophage — a promising alternative measure for bacterial biofilm control. Infect. Drug Resist. 2021;14:205–17. DOI: https://doi.org/10.2147/IDR.S290093
- Kovacs C.J., Rapp E.M., McKenzie S.M., et al. Disruption of biofilm by bacteriophages in clinically relevant settings. Mil. Med. 2024;189(5-6):e1294–302. DOI: https://doi.org/10.1093/milmed/usad385
- Mayorga-Ramos A., Carrera-Pacheco S.E., Barba-Ostria C., et al. Bacteriophage-mediated approaches for biofilm control. Front. Cell Infect. Microbiol. 2024;14:1428637. DOI: https://doi.org/10.3389/fcimb.2024.1428637
- Zurabov F., Glazunov E., Kochetova T., et al. Bacteriophages with depolymerase activity in the control of antibiotic resistant Klebsiella pneumoniae biofilms. Sci. Rep. 2023;13:15188. DOI: https://doi.org/10.1038/s41598-023-42505-3
- Pallavali R.R., Degati V.L., Narala V.R., et al. Lytic bacteriophages against bacterial biofilms formed by multidrug-resistant Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus isolated from burn wounds. Phage (New Rochelle). 2021;2(3):120–30. DOI: https://doi.org/10.1089/phage.2021.0004
- Chen W., Han L.M., Chen X.Z., et al. Engineered endolysin of Klebsiella pneumoniae phage is a potent and broad-spectrum bactericidal agent against "ESKAPEE" pathogens. Front. Microbiol. 2024;15:1397830. DOI: https://doi.org/10.3389/fmicb.2024.1397830
- Shahed-Al-Mahmud M., Roy R., Sugiokto F.G., et al. Phage φAB6-borne depolymerase combats Acinetobacter baumannii biofilm formation and infection. Antibiotics (Basel). 2021;10(3):279. DOI: https://doi.org/10.3390/antibiotics10030279
- Fu W., Forster T., Mayer O., et al. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob. Agents Chemother. 2010;54(1):397–404. DOI: https://doi.org/10.1128/AAC.00669-09
- Curtin J.J., Donlan R.M. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2006;50(4):1268–75. DOI: https://doi.org/10.1128/AAC.50.4.1268-1275.2006
- Cieślik M., Bagińska N., Górski A., et al. Animal models in the evaluation of the effectiveness of phage therapy for infections caused by gram-negative bacteria from the ESKAPE group and the reliability of its use in humans. Microorganisms. 2021;9(2):206. DOI: https://doi.org/10.3390/microorganisms9020206
- Mehmood Khan F., Manohar P., Singh Gondil V., et al. The applications of animal models in phage therapy: an update. Hum. Vaccin. Immunother. 2023;19(1):2175519. DOI: https://doi.org/10.1080/21645515.2023.2175519
- Singh A.N., Singh A., Nath G. Evaluation of bacteriophage cocktail on urinary tract infection caused by colistin-resistant Klebsiella pneumoniae in mice model. J. Glob. Antimicrob. Resist. 2024;39:41–53. DOI: https://doi.org/10.1016/j.jgar.2024.07.019
Supplementary files