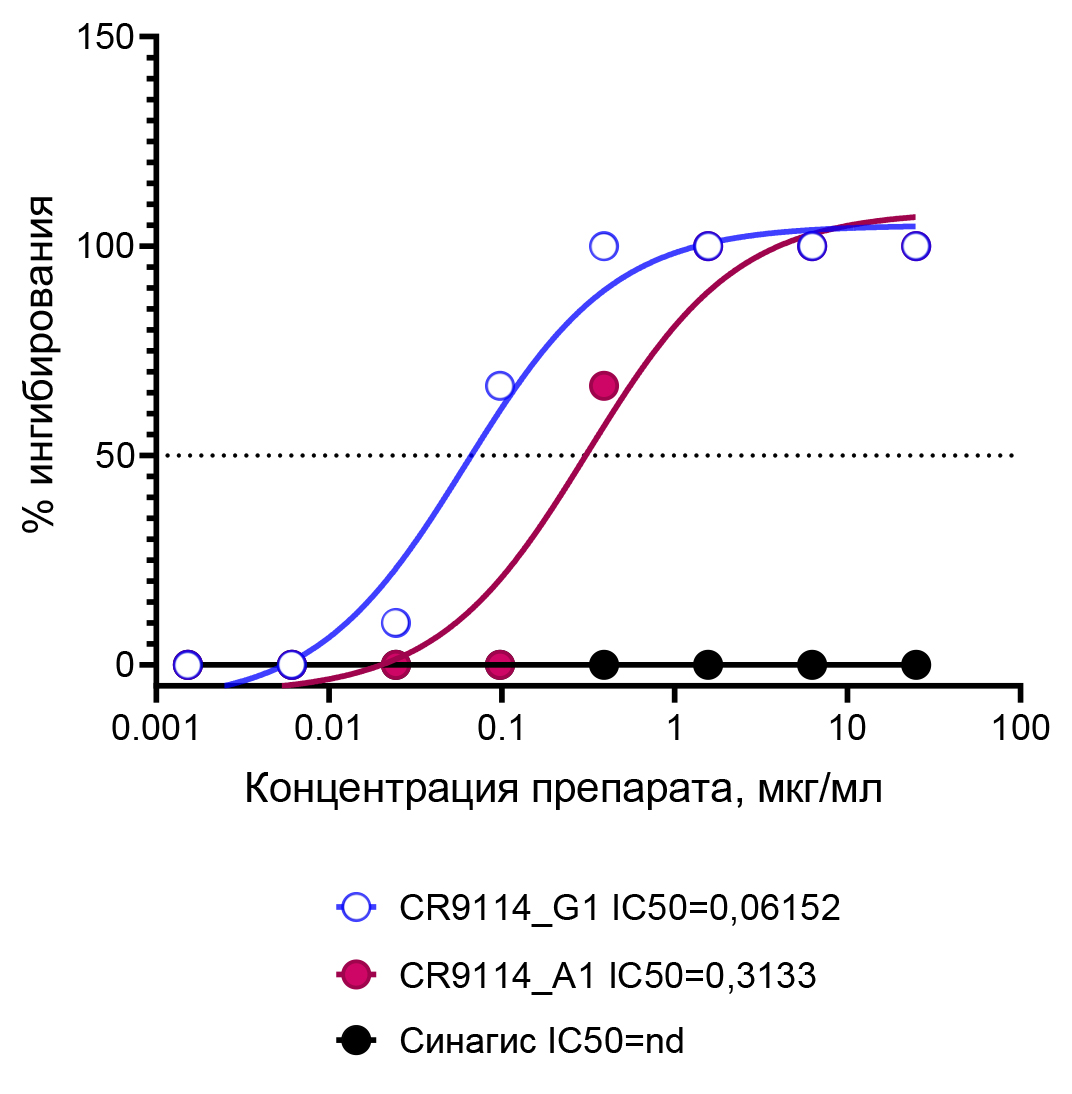
Protective activity of CR9114 universal antibody isotypes against influenza A virus in vivo
- Authors: Romanovskaya-Romanko E.A.1, Plotnikova M.A.1, Oleynik V.A.1, Shaldzhyan A.A.1, Monakhova V.S.1, Balabashin D.S.2, Toporova V.A.1,2, Aliev T.K.2,3, Klotchenko S.A.1
-
Affiliations:
- Smorodintsev Research Institute of Influenza
- Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences
- Lomonosov Moscow State University
- Issue: Vol 102, No 6 (2025)
- Pages: 783-793
- Section: ORIGINAL RESEARCHES
- URL: https://medbiosci.ru/0372-9311/article/view/381682
- DOI: https://doi.org/10.36233/0372-9311-767
- EDN: https://elibrary.ru/EDEOBG
- ID: 381682
Cite item
Abstract
Introduction. Influenza can cause diseases of varying severity, sometimes leading to hospitalization or death. One of the most promising strategies aimed at reducing morbidity and preventing the risks of severe consequences of infection is the use of broad-spectrum antibodies that provide effective protection against infection with seasonal strains.
The aim of the study was to evaluate the protective activity of CR9114 antibodies of the IgG1 and IgA1 isotypes when administered systemically and locally against experimental influenza infection in mice.
Materials and methods. The recombinant antibodies CR9114 of IgG1 or IgA1 isotypes were administered intranasally to BALB/c mice at a dose of 100 or 20 μg 24 hours before infection with influenza virus A/California/07/09 (H1N1)pdm09 virus at a dose of 10 MLD50 (prophylactic regimen) and/or 24 hours after infection (therapeutic regimen). Body weight dynamics were assessed and mortality was recorded in the animals for 14 days after infection.
Results. Intranasal administration of IgG1 or IgA1 isotype antibodies in the therapeutic-prophylactic regimen led to a decrease in viral load in the respiratory tract tissues of infected mice. At the same time, parenteral administration of IgG (but not IgA) also reduced the virus titer in the nasal passages (but not in the lungs) of mice. It was demonstrated that prophylactic administration of IgG1 or IgA1 antibodies provides complete protection against lethal influenza infection.
Conclusion. Intranasal prophylactic administration of human neutralizing antibodies CR9114 of IgG1 or IgA1 isotypes provides 100% survival of mice in lethal infection with influenza A/California/07/09 (H1N1)pdm09 virus. At the same time, Fc fragments of immunoglobulins of different isotypes, responsible for effector functions, appear to influence the degree of antiviral protection.
About the authors
Ekaterina A. Romanovskaya-Romanko
Smorodintsev Research Institute of Influenza
Email: romromka@yandex.ru
ORCID iD: 0000-0001-7560-398X
Cand. Sci. (Biol.), leading researcher, Laboratory of Vector Vaccines
Russian Federation, St. PetersburgMarina A. Plotnikova
Smorodintsev Research Institute of Influenza
Email: biomalinka@mail.ru
ORCID iD: 0000-0001-8196-3156
Cand. Sci. (Biol.), seniour researcher, Laboratory of Vector Vaccines
Russian Federation, St. PetersburgVeronika A. Oleynik
Smorodintsev Research Institute of Influenza
Email: working.lyutik@gmail.com
ORCID iD: 0009-0005-3081-0463
laboratory researcher, Laboratory of immunobiological technologies
Russian Federation, St. PetersburgAram A. Shaldzhyan
Smorodintsev Research Institute of Influenza
Email: shaldzhyan@yandex.ru
ORCID iD: 0000-0002-8646-6252
laboratory researcher, Laboratory of genetic engineering and recombinant protein expression
Russian Federation, St. PetersburgVarvara S. Monakhova
Smorodintsev Research Institute of Influenza
Email: varvara.bio@gmail.com
ORCID iD: 0009-0002-9519-5316
Cand. Sci. (Biol.), researcher, Laboratory of immunobiological technologies
Russian Federation, St. PetersburgDmitry S. Balabashin
Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences
Email: dbalabashin@mail.ru
ORCID iD: 0000-0002-7627-0600
Cand. Sci. (Biol.), junior researcher, Laboratory of protein engineering
Russian Federation, MoscowViktoriya A. Toporova
Smorodintsev Research Institute of Influenza; Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences
Email: toporova.viktorija@gmail.com
ORCID iD: 0000-0002-7450-7096
laboratory researcher, Laboratory of immunobiological technologies, Smorodintsev Research Institute of Influenza; researcher, Laboratory of protein engineering, Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry
Russian Federation, St. Petersburg; MoscowTeymur K. Aliev
Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences; Lomonosov Moscow State University
Email: ta12345@list.ru
ORCID iD: 0000-0002-1753-9614
Cand. Sci. (Chem.), Deputy Head, Competence Center of the National Technological Initiative, Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry; researcher, Department of chemical enzymology, Lomonosov Moscow State University
Russian Federation, Moscow; MoscowSergey A. Klotchenko
Smorodintsev Research Institute of Influenza
Author for correspondence.
Email: fosfatik@mail.ru
ORCID iD: 0000-0003-0289-6560
Cand. Sci. (Biol.), Head, Laboratory of immunobiological technologies
Russian Federation, St. PetersburgReferences
- Bates A., Power C.A. David vs. Goliath: The structure, function, and clinical prospects of antibody fragments. Antibodies. 2019;8(2):28. DOI: https://doi.org/10.3390/antib8020028
- Klasse P.J. Neutralization of virus infectivity by antibodies: old problems in new perspectives. Adv. Biol. 2014;2014:1–24. DOI: https://doi.org/10.1155/2014/157895
- Dilillo D.J., Tan G.S., Palese P., Ravetch J.V. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcR interactions for protection against influenza virus in vivo. Nat. Med. 2014;20(2):143–51. DOI: https://doi.org/10.1038/nm.3443
- Ben Mkaddem S., Benhamou M., Monteiro R.C. Understanding Fc receptor involvement in inflammatory diseases: from mechanisms to new therapeutic tools. Front. Immunol. 2019;10:811. DOI: https://doi.org/10.3389/fimmu.2019.00811
- Клотченко С.А., Романовская-Романько Е.А., Плотникова М.А. и др. Разработка и исследование вируснейтрализующей активности рекомбинантного человеческого антитела к F-гликопротеину респираторно-синцитиального вируса. Журнал микробиологии, эпидемиологии и иммунобиологии. 2024;101(6):735–47. Klotchenko S.A., Romanovskaya-Romanko E.A., Plotnikova M.A., et al. Development and evaluation of a recombinant monoclonal human antibody with virus-neutralizing activity against the F glycoprotein of respiratory syncytial virus. Journal of Microbiology, Epidemiology and Immunobiology. 2024;101(6):735–47. DOI: https://doi.org/10.36233/0372-9311-611 EDN: https://elibrary.ru/zkqvtw
- Cottignies-Calamarte A., Tudor D., Bomsel M. Antibody Fc-chimerism and effector functions: When IgG takes advantage of IgA. Front. Immunol. 2023;14:1037033. DOI: https://doi.org/10.3389/fimmu.2023.1037033
- Li B., Xu L., Tao F., et al. Simultaneous exposure to FcγR and FcaR on monocytes and macrophages enhances antitumor activity in vivo. Oncotarget. 2017;8(24):39356–66. DOI: https://doi.org/10.18632/oncotarget.17000
- Bohländer F. A new hope? Possibilities of therapeutic IgA antibodies in the treatment of inflammatory lung diseases. Front. Immunol. 2023;14:1127339. DOI: https://doi.org/10.3389/fimmu.2023.1127339
- Corti D., Agatic G., Bianchi S., et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333(6044):850–6. DOI: https://doi.org/10.1126/science.1205669
- Momont C., Dang H.V., Zatta F., et al. A pan-influenza antibody inhibiting neuraminidase via receptor mimicry. Nature. 2023;618(7965):590–7. DOI: https://doi.org/10.1038/s41586-023-06136-y
- Biswas M., Yamazaki T., Chiba J., Akashi-Takamura S. Broadly neutralizing antibodies for influenza: рassive immunotherapy and intranasal vaccination. Vaccines. 2020;8(3):424. DOI: https://doi.org/10.3390/vaccines8030424
- Dreyfus C., Laursen N.S., Kwaks T., et al. Highly conserved protective epitopes on influenza B viruses. Science. 2012; 337(6100):1343–8. DOI: https://doi.org/10.1126/science.1222908
- Beukenhorst A.L., Frallicciardi J., Rice K.L., et al. A pan-influenza monoclonal antibody neutralizes H5 strains and prophylactically protects through intranasal administration. Sci. Rep. 2024;14(1):3818. DOI: https://doi.org/10.1038/s41598-024-53049-5
- Bairoch A., Apweiler R., Wu C.H., et al. The Universal Protein Resource (UniProt). Nucleic Acids Res. 2005;33(Database issue):D154–9. DOI: https://doi.org/10.1093/nar/gki070
- Johnson S., Oliver C., Prince G.A., et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997;176(5):1215–24. DOI: https://doi.org/10.1086/514115
- Кривицкая В.З., Петрова Е.Р., Сорокин Е.В. и др. Получение и характеристика моноклональных антител, специфичных к респираторно-синцитиальному вирусу. Биотехнология. 2016;32(1):65–75. Krivitskaya V.Z., Petrova E.R., Sorokin E.V., et al. Design and characteristics of monoclonal antibodies specific to respiratory syncytial virus. Biotechnology. 2016;32(1):65–75. EDN: https://elibrary.ru/vvzkst
- Ларионова Н.В., Киселева И.В., Баженова Е.А. и др. Влияние биологических свойств сезонных вирусов гриппа на эффективность подготовки штаммов живой гриппозной вакцины. Журнал микробиологии, эпидемиологии и иммунобиологии. 2019;(5):24–34. Larionova N.V., Kiseleva I.V., Bazhenova E.A., et al. The influence of seasonal influenza viruses biological features on the effectiveness of development strains for live influenza vaccine. Journal of Microbiology, Epidemiology and Immunobiology. 2019;(5):24–34. DOI: https://doi.org/10.36233/0372-9311-2019-5-24-34 EDN: https://elibrary.ru/rucdbf
- de Sousa-Pereira P., Woof J.M. IgA: structure, function, and developability. Antibodies (Basel). 2019;8(4):57. DOI: https://doi.org/10.3390/antib8040057
- Beukenhorst A.L., Rice K.L., Frallicciardi J., et al. Intranasal administration of a panreactive influenza antibody reveals Fc-independent mode of protection. Sci. Rep. 2025;15(1):10309. DOI: https://doi.org/10.1038/s41598-025-94314-5
- Schroeder H.W., Cavacini L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2012;125(202):S41–52. DOI: https://doi.org/10.1016/j.jaci.2009.09.046
- Maurer M.A., Meyer L., Bianchi M., et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep. 2018;23(1):90–9. DOI: https://doi.org/10.1016/j.celrep.2018.03.027
- Lo M., Kim H.S., Tong R.K., et al. Effector-attenuating substitutions that maintain antibody stability and reduce toxicity in mice. J. Biol. Chem. 2017;292(9):3900–8. DOI: https://doi.org/10.1074/jbc.M116.767749
- Kallewaard N.L., Corti D., Collins P.J., et al. Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell. 2016;166(3):596–608. DOI: https://doi.org/10.1016/j.cell.2016.05.073
Supplementary files